Bone Health: Examining the Evidence
Bone health is a complex structure that necessitates communication and synchronicity between various organs and tissues. Multiple factors influence our habits surrounding nutrient intake and exercise, which are known determinants of our skeletal health. (3)
By weight, bone tissue is made up of about 60% inorganic calcium hydroxyapatite salt, 30% organic synthesized material, such as type 1 collagen (roughly 90%) and non-collagenous proteins (roughly 10%), and 10% percent water.
(15) The complex nature of bone is supportive to our health physically by providing structure to our skeleton and regulating physiology and electrolyte balance.
Bone mass development: osteoporosis and fracture risk
Bone remodeling, scientifically referred to as osteoblastic and osteoclastic activity, is the name given to describe the balancing effect of bone health over a lifetime, and it is this aspect that determines osteoporosis and fracture risk. Bone turnover reflects the process of osteoclastic activity that drives calcium from the bone to the blood. (6)
Osteoporosis has been coined by Osteoporosis Canada as a pediatric disease with geriatric consequences. Peak bone mass is achieved between the ages of 16 to 20 in young women and 20 to 25 years in young men.
The statistics for developing osteoporosis are alarmingly high; one in three for women and one in five for men will develop osteoporosis in their lifetimes. In post-menopausal women, bone loss can occur at a rate of two to three percent per year. (32) The all-time risk of developing osteoporosis is 50% if the body does not meet its calcium requirements. (30)
Apart from dietary calcium, research has identified a number of factors impacting bone mass development, which include engaging daily physical activity from adolescence through adulthood (particularly for adolescent boys), consuming fruits (particularly for young girls), and consuming both fruits and vegetables (particularly for young boys). (6)(27)(43)
One in three for women and one in five for men develop osteoporosis in their lifetime.
Bone fracture risk factors
Fracture risk is determined by several factors, such as personal adult fracture history, having a first degree relative with a fragility fracture, smoking, and using corticosteroids for more than three months. (42) The Study of Osteoporotic Fractures Research Group which followed 9704 postmenopausal women identified these additional risk factors:
- Benzodiazepine use
- Height
- History of maternal hip fracture
- Low body weight
- Low bone mineral density (BMD)
- Poor depth perception
- Poor health
- Previous fracture
- Previous hyperthyroidism
- Tachycardia (12)
A T-Score on dual-energy x-ray absorptiometry (DEXA) between -1 and -2.5 is considered high risk and necessitates treatment. (42) Bone loss can start as early as 45 years of age, and fractures are responsible for approximately 44% of elderly being admitted to nursing homes in the US and longer hospital days than other illnesses, and therefore, is a topic worth addressing. (30)
3 key nutrients for bone health
Calcium, vitamin D, and vitamin K play essential roles in supporting bone health and reducing the risk of osteoporosis and fractures.
Calcium and bone health
Dietary calcium is essential to make up the bone matrix, and consensus suggests that an adult in North America requires about 1,000 mg of calcium daily. Several factors play a role in calcium metabolism. While the biochemistry of calcium metabolism is beyond the scope of this short review, Table 1 provides a quick reference to the hormones involved in maintaining serum calcium levels and Table 2 provides an overview of the dietary influences in calcium homeostasis and bone health. Potential factors related to malabsorption of calcium, such as low stomach acidity or inflammation of the gastrointestinal tract, are important considerations when considering calcium intake. (30)
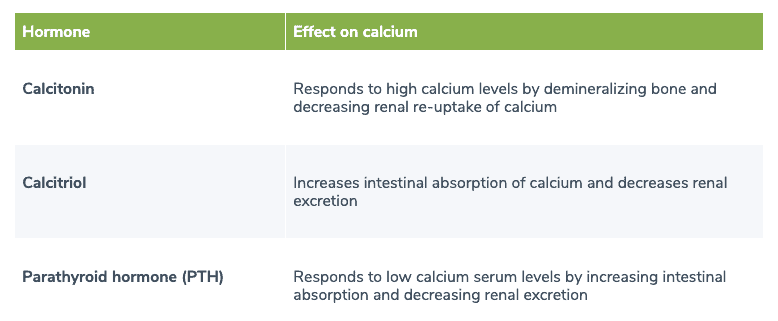
Table 1. Hormones involved in calcium homeostasis
Calcitonin, calcitriol, and PTH are the hormones involved in calcium homeostasis. (1)
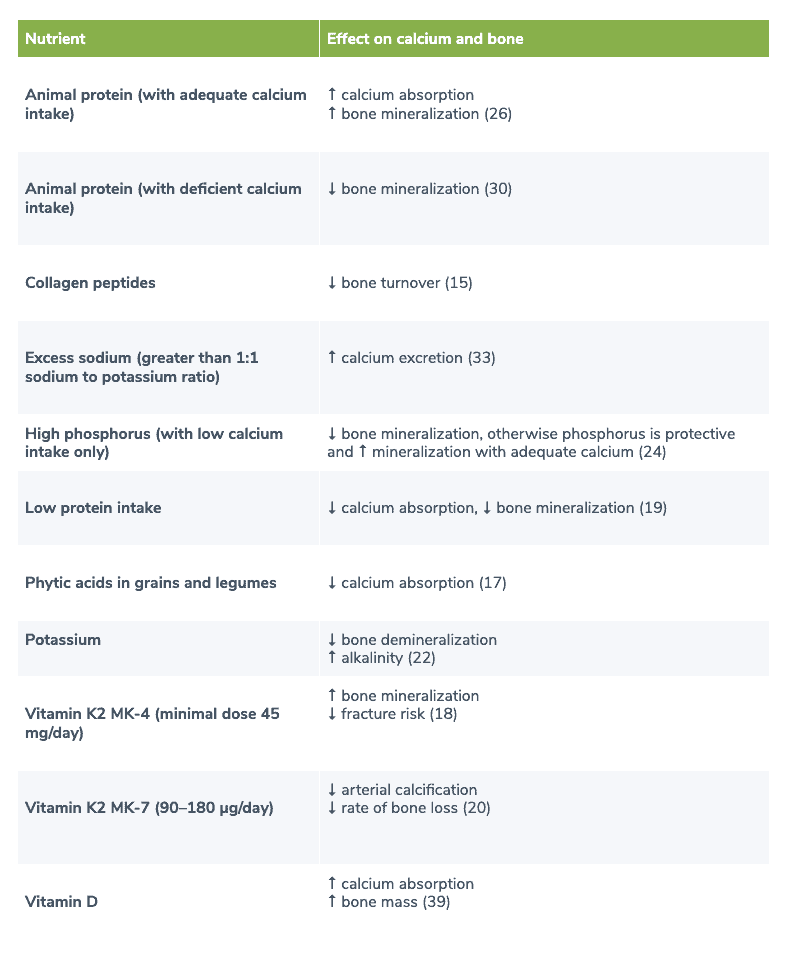
Table 2. Nutrients involved in calcium homeostasis and bone mineralization
Several nutrients are involved in calcium homeostasis and bone mineralization.
Supplemental calcium
Concerns regarding supplemental calcium were introduced by the Women’s Health Initiative study that uncovered a possible connection with supplemental (not dietary) calcium intake with death from heart attack and ischemic heart disease. (28) A second important study, a meta-analysis of 11 randomized controlled trials (RCTs) found that calcium supplementation, without vitamin D, contributed to death caused by myocardial infarction. (4)
The results from these studies led to significant media attention surrounding supplemental calcium and the risk of death. Nevertheless, soon after these results were made public, concerns were thwarted as re-analysis determined that these conclusions were not definitive. Cardiovascular disease was not the primary endpoint measure of these studies, and the cause and effect conclusions could not be significant because the phenomena was observed, not carried out by way of intervention.
An updated remark was released in the Annals of Internal Medicine in 2016 that guides clinicians on the safe use of supplemental calcium when dietary means are insufficient, without increasing risk of cardiovascular disease. (23)
Calcium supplement guidelines
Because large intakes of calcium per dose may increase the risk of arterial calcification, it is suggested to achieve daily requirements of calcium through dietary means, and if that is not possible, through supplementation of calcium as smaller boluses in divided doses, with co-factors that reduce peripheral calcium such as vitamin D and vitamin K2. (30)(45)
Forms of calcium
The evidence regarding which calcium is best for absorption and which is best for clinical effect is under investigation.
Microcrystalline hydroxyapatite
Microcrystalline hydroxyapatite (MCHA), the crystalized form of calcium naturally found in the bone matrix, has been under investigation. Why this form of calcium is so intriguing is based on a few ideas.
First, it is thought that the collagenous and non-collagenous proteins that make up bone may be implicated in bone remodeling, remineralization, and the attachment to bone.
(15) Secondly, it has been demonstrated in human research that ingestion of MCHA produces less of an acute spike in serum calcium levels compared to soluble calcium supplements, an important consideration for cardiovascular disease prevention concerning supplemental calcium. (5)
Calcium carbonate and calcium citrate
One single serving study of calcium on 23 healthy premenopausal women found that calcium carbonate was better absorbed than calcium citrate. (44) On the other hand, another author describes more precise indications that calcium carbonate is best absorbed when taken with a meal, and that calcium citrate, to be taken without food, is better indicated for people with low stomach acidity or who take proton pump inhibitor medications. (38)
One hundred post-menopausal women (mean age of 71 years old) were randomized to receive either calcium citrate, calcium carbonate, or one of two MCHA preparations or placebo. After eight hours of ingestion and after the three-month mark, ionized and total serum calcium were increased and remained increased in the citrate and carbonate groups.
The MCHA had less of a spike compared to the citrate and carbonate groups. All forms of calcium were able to reduce PTH and favorably affect bone turnover markers at three months. The authors discuss the low bioavailability of calcium in MCHA could possibly be due to the extra step of hydrolysis needed to break the complex, which may lead to less calcium in circulation. (5)
Bioavailability vs. clinical effect: the conundrum
Interestingly, MCHA that has been shown to produce less of a spike in serum calcium and be potentially less bioavailable has also been demonstrated to be more clinically effective when compared to calcium carbonate. (7)(8)(9)(10)(34)(35) Details of three clinical studies and one meta-analysis are presented below.
A 2009 meta-analysis of six RCTs, with no significant degree of heterogeneity, was in favor for the use of ossein hydroxyapatite calcium (OHC) to increase bone mineral density (an increase by 1.02%; P<0.00001) compared to calcium carbonate (CC). (7)
A 2011 randomized, open-label, parallel group, controlled, prospective study investigated the effects of 830 mg OHC twice daily (providing 712 mg elemental calcium daily) and 500 mg CC twice daily (providing 1000 mg elemental calcium daily), along with vitamin D in 120 women (54 completed the study) over three years.
Increases in serum osteocalcin were greater in the OHC group compared to CC (P<0.05), and levels continued to increase from year two to year three (P<0.05). Results were not statistically significant in the CC group. Bone mineral density at the lumbar spine and femoral neck between baseline and year three were -1.1% and 2.5% for OHC and -2.3% and 1.2% for CC, respectively. (10)
Another 2015 randomized, open-label, parallel group, controlled, prospective study investigated the effect of 1200 mg per day of CC (n=38) or 1660 mg per day of OHC (n=36) on pain and quality of life associated with osteopenia for six months in 74 postmenopausal women.
Compared to CC, OHC was significantly better at controlling back and knee pain, including exercise-induced pain. Quality of life, as reported by physical, social, and emotional/mental components, demonstrated a significant improvement for OHC and no significant change for CC. (9)
A 2020 prospective, non-randomized, comparative, open-label trial including 851 peri-menopausal women with BMD T-score ≥ -2 standard deviation (SD) were given either 712 mg per day of OHC or 1000 mg per day of CC for three years.
BMD was evaluated at 18 and 36 months. BMD of the lumbar spine (L2-L4) remained unchanged in the OHC group and decreased by -3.1% in the CC group. These differences were statistically significant. Additionally, CC had more incidences of gastrointestinal adverse effects. (8)
The mechanism of the anabolic effect of OHC or MCHA is not fully understood. Possible mechanisms include the presence of organic proteins found in MCHA that have been shown in vitro to be mitogenic for bone cell growth. (36)(37)
Safety of MCHA
The safety of using MCHA has not been researched among all patient cohorts or demographics. However, based on the above studies and another positive trial of four years, MCHA appears to be safe and well tolerated, with minimal side effects, except for mild constipation in approximately 3% of individuals. (16) No incidence of kidney stones has been reported.
Calcium, vitamin D, and vitamin K are essential nutrients for bone health.
Vitamin K2 and bone health
Vitamin K2 has been shown to protect against bone loss and reduce the risk of fractures, particularly when used in conjunction with vitamin D and calcium. A large and statistically rigorous meta-analysis of seven trials examining the use of vitamin K (phytonadione and menaquinone-4) in Japanese women concluded that high vitamin K2 levels were associated with reduced vertebral fractures by approximately 60%, hip fractures by 77%, and all nonvertebral fractures by approximately 81%. (11)
Vitamin K2 MK-4
In a review of eight RCTs, vitamin K2 MK-4 (as a synthetic drug) at a minimum effective dose of 45 mg per day was found to decrease serum under-caboxylated osteocalcin (ucOC), reduce the incidence of fractures, modestly increase lumbar spine BMD, and increase efficacy with alendronate in postmenopausal women with osteoporosis. (18)
Vitamin K2 MK-7
All-trans menaquione-7 (MK-7) has the geometric isomerization of natural MK-7 found in fermented natto (soybean). It has been demonstrated in studies to be bioequivalent to naturally sourced MK-7 at a cost-effective dose. (29)
An RCT of 311 community dwelling Chinese men and women aged 50 to 75 years received either a placebo, vitamin K2 MK-7, or vitamin K2 MK-7 in conjunction with vitamin D and calcium for one year. Bone loss of the femoral neck was reduced in women (only) in both the vitamin K2 group and the vitamin K2 with calcium and vitamin D group (P=0.006). It was found that vitamin K2, even alone, at 90 mcg per day produced a significant effect on decreasing bone loss in women. (46)
A double-blind, placebo-controlled study investigated the effects of vitamin K2 MK-7 (180 mcg per day) (n=120) on arterial stiffness in healthy postmenopausal women compared to placebo (n=124) for three years. MK-7 was found to improve arterial stiffness while having no important effects on endothelial dysfunction. (20)
The same study investigated the effects on bone mineral content. A significant reduction in the decline of age-related bone mineral density and bone mineral content at the lumbar spine and femoral neck was observed in the MK-7 group compared to placebo. MK-7 reduced the loss of vertebral height of the lower thoracic region. It also increased serum levels of active/carboxylated osteocalcin and lowered levels of inactive/undercarboxylated osteocalcin. (21)
Currently, there is a two-year multi-centre, double-blind placebo-controlled trial investigating the effects of MK-7 versus placebo in 400 men with substantial aortic valve calcification. (25)
Safety of vitamin K2
Vitamin K2 MK-7 has been shown to increase coagulation; therefore, caution is warranted. (41) The quality and proper testing of vitamin K2 MK-7 products are important considerations when selecting K2. A study found dietary supplements of MK-7 to contain a high degree of variability in dose compared to what is on the label. The study also found evidence of the cis-isomer of MK-7, which can have potential toxic effects. (40) MK-7 is highly unstable in suspension and, therefore, should be taken as a stand-alone supplement. (31)
Collagen and bone health
A recent randomized prospective study compared the effects of collagen peptides (CPs) combined with 500 mg of elemental calcium and 400 IU of vitamin D with the same dose of elemental calcium and vitamin D without CPs.
Within three months, markers of bone turnover, procollagen type I N-terminal propeptide, and C-terminal telopeptide of collagen I decreased in the CPs group by 13.1% (p<0.001) and 11.4% (P=0.058), respectively. No change was observed in the group without CPs. (2)
Conversely, a randomized, double-blind controlled study investigating the effects of 10 g per day of collagen hydrolysates (n=35) vs. a placebo (n=35) for 24 weeks found no effect on bone remodeling markers in post-menopausal women. (13) Differences attributed between the two studies could be due to the absence of calcium and vitamin D in the latter study.
Finally, authors from the first study investigated the use of collagen-calcium chelate (CCC) long-term. Thirty-nine post-menopausal osteopenic women were randomly assigned to either 5 g of CCC with 500 mg of elemental calcium and 200 IU of vitamin D or 500 mg of elemental calcium and 200 IU of vitamin D without CCC daily for 12 months.
The CCC group demonstrated a significant reduction in the loss of BMD compared to the control group who completed the study (CCC: -1.33% and -0.33% vs. control P=0.026). The CCC group had higher bone-specific alkaline phosphatase/TRAP5b ratio (P<0.05) and lower levels of bone turnover markers (P<0.05) compared to control at six months. (14)
The bottom line
In order to maintain bone health and reduce osteoporosis and fracture risk, using supplemental calcium when dietary intake is not sufficient is suggested, along with a comprehensive plan for calcium homeostasis.
When increased calcium intake is necessary for a therapeutic effect and to reduce fracture risk, supplementing the diet with vitamin K2 and collagen may be considered alongside calcium and other bone-supportive nutrients, such as magnesium and vitamin D.
Source(s) Fullscript
REFERENCES
- American Bone Health. (2019, May 4). How the Body Maintains Calcium Levels.
- Argyrou, C., Karlafti, E., Lampropoulou-Adamidou, K., Tournis, S., Makris, K., Trovas, G., Dontas, I., & Triantafyllopoulos, I. K. (2020). Effect of calcium and vitamin D supplementation with and without collagen peptides on bone turnover in postmenopausal women with osteopenia. Journal of musculoskeletal & neuronal interactions, 20(1), 12–17.
- Bielemann, R. M., Martinez-Mesa, J., & Gigante, D. P. (2013). Physical activity during life course and bone mass: a systematic review of methods and findings from cohort studies with young adults. BMC musculoskeletal disorders, 14, 77.
- Bolland, M. J., Avenell, A., Baron, J. A., Grey, A., MacLennan, G. S., Gamble, G. D., & Reid, I. R. (2010). Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ (Clinical research ed.), 341, c3691.
- Bristow, S. M., Gamble, G. D., Stewart, A., Horne, L., House, M. E., Aati, O., Mihov, B., Horne, A. M., & Reid, I. R. (2014). Acute and 3-month effects of microcrystalline hydroxyapatite, calcium citrate and calcium carbonate on serum calcium and markers of bone turnover: a randomised controlled trial in postmenopausal women. The British journal of nutrition, 112(10), 1611–1620.
- Buckwalter, J. A., & Cooper, R. R. (1987). Bone structure and function. Instructional course lectures, 36, 27–48.
- Castelo-Branco, C., Ciria-Recasens, M., Cancelo-Hidalgo, M. J., Palacios, S., Haya-Palazuelos, J., Carbonell-Abelló, J., Blanch-Rubió, J., Martínez-Zapata, M. J., Manasanch, J., & Pérez-Edo, L. (2009). Efficacy of ossein-hydroxyapatite complex compared with calcium carbonate to prevent bone loss: a meta-analysis. Menopause (New York, N.Y.), 16(5), 984–991.
- Castelo-Branco, C., Cancelo Hidalgo, M. J., Palacios, S., Ciria-Recasens, M., Fernández-Pareja, A., Carbonell-Abella, C., Manasanch, J., & Haya-Palazuelos, J. (2020). Efficacy and safety of ossein-hydroxyapatite complex versus calcium carbonate to prevent bone loss. Climacteric : the journal of the International Menopause Society, 23(3), 252–258.
- Castelo-Branco, C., Dávila, J., Alvarez, L., & Balasch, J. (2015). Comparison of the effects of calcium carbonate and ossein-hydroxyapatite complex on back and knee pain and quality of life in osteopenic perimenopausal women. Maturitas, 81(1), 76–82.
- Ciria-Recasens, M., Blanch-Rubió, J., Coll-Batet, M., Del Pilar Lisbona-Pérez, M., Díez-Perez, A., Carbonell-Abelló, J., Manasanch, J., & Pérez-Edo, L. (2011). Comparison of the effects of ossein-hydroxyapatite complex and calcium carbonate on bone metabolism in women with senile osteoporosis: a randomized, open-label, parallel-group, controlled, prospective study. Clinical drug investigation, 31(12), 817–824.
- Cockayne, S., Adamson, J., Lanham-New, S., Shearer, M. J., Gilbody, S., & Torgerson, D. J. (2006). Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Archives of internal medicine, 166(12), 1256–1261.
- Cummings, S. R., Nevitt, M. C., Browner, W. S., Stone, K., Fox, K. M., Ensrud, K. E., Cauley, J., Black, D., & Vogt, T. M. (1995). Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. The New England journal of medicine, 332(12), 767–773.
- Cúneo, F., Costa-Paiva, L., Pinto-Neto, A. M., Morais, S. S., & Amaya-Farfan, J. (2010). Effect of dietary supplementation with collagen hydrolysates on bone metabolism of postmenopausal women with low mineral density. Maturitas, 65(3), 253–257.
- Elam, M. L., Johnson, S. A., Hooshmand, S., Feresin, R. G., Payton, M. E., Gu, J., & Arjmandi, B. H. (2015). A calcium-collagen chelate dietary supplement attenuates bone loss in postmenopausal women with osteopenia: a randomized controlled trial. Journal of medicinal food, 18(3), 324–331.
- Feng X. (2009). Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Current chemical biology, 3(2), 189–196.
- Fernández-Pareja, A., Hernández-Blanco, E., Pérez-Maceda, J. M., Riera Rubio, V. J., Palazuelos, J. H., & Dalmau, J. M. (2007). Prevention of osteoporosis: four-year follow-up of a cohort of postmenopausal women treated with an ossein-hydroxyapatite compound. Clinical drug investigation, 27(4), 227–232.
- Gupta, R. K., Gangoliya, S. S., & Singh, N. K. (2015). Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. Journal of food science and technology, 52(2), 676–684.
- Iwamoto J. (2014). Vitamin K₂ therapy for postmenopausal osteoporosis. Nutrients, 6(5), 1971–1980.
- Kerstetter, J. E., O’Brien, K. O., & Insogna, K. L. (2003). Low protein intake: the impact on calcium and bone homeostasis in humans. The Journal of nutrition, 133(3), 855S–861S.
- Knapen, M. H., Braam, L. A., Drummen, N. E., Bekers, O., Hoeks, A. P., & Vermeer, C. (2015). Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thrombosis and haemostasis, 113(5), 1135–1144.
- Knapen, M. H., Drummen, N. E., Smit, E., Vermeer, C., & Theuwissen, E. (2013). Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 24(9), 2499–2507.
- Kong, S. H., Kim, J. H., Hong, A. R., Lee, J. H., Kim, S. W., & Shin, C. S. (2017). Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES) (2008-2011). Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 28(5), 1577–1585.
- Kopecky, S. L., Bauer, D. C., Gulati, M., Nieves, J. W., Singer, A. J., Toth, P. P., Underberg, J. A., Wallace, T. C., & Weaver, C. M. (2016). Lack of Evidence Linking Calcium With or Without Vitamin D Supplementation to Cardiovascular Disease in Generally Healthy Adults: A Clinical Guideline From the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Annals of internal medicine, 165(12), 867–868.
- Lee, A. W., & Cho, S. S. (2015). Association between phosphorus intake and bone health in the NHANES population. Nutrition journal, 14, 28.
- Lindholt, J. S., Frandsen, N. E., Fredgart, M. H., Øvrehus, K. A., Dahl, J. S., Møller, J. E., Folkestad, L., Urbonaviciene, G., Becker, S. W., Lambrechtsen, J., Auscher, S., Hosbond, S., Alan, D. H., Rasmussen, L. M., Gerke, O., Mickley, H., & Diederichsen, A. (2018). Effects of menaquinone-7 supplementation in patients with aortic valve calcification: study protocol for a randomised controlled trial. BMJ open, 8(8), e022019.
- Mangano, K. M., Sahni, S., & Kerstetter, J. E. (2014). Dietary protein is beneficial to bone health under conditions of adequate calcium intake: an update on clinical research. Current opinion in clinical nutrition and metabolic care, 17(1), 69–74.
- McGartland, C. P., Robson, P. J., Murray, L. J., Cran, G. W., Savage, M. J., Watkins, D. C., Rooney, M. M., & Boreham, C. A. (2004). Fruit and vegetable consumption and bone mineral density: the Northern Ireland Young Hearts Project. The American journal of clinical nutrition, 80(4), 1019–1023.
- Michaëlsson, K., Melhus, H., Warensjö Lemming, E., Wolk, A., & Byberg, L. (2013). Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. BMJ (Clinical research ed.), 346, f228.
- Møller, M., Gjelstad, I., Baksaas, I., Grande, T., Aukrust, I. R., & Drevon, C. A. (2017). Bioavailability and Chemical/Functional Aspects of Synthetic MK-7 vs Fermentation-Derived MK-7 in Randomised Controlled Trials. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition, 87(5-6), 1–15.
- O’Keefe, J. H., Bergman, N., Carrera-Bastos, P., Fontes-Villalba, M., DiNicolantonio, J. J., & Cordain, L. (2016). Nutritional strategies for skeletal and cardiovascular health: hard bones, soft arteries, rather than vice versa. Open heart, 3(1), e000325.
- Orlando, P., Silvestri, S., Marcheggiani, F., Cirilli, I., & Tiano, L. (2019). Menaquinone 7 Stability of Formulations and Its Relationship with Purity Profile. Molecules (Basel, Switzerland), 24(5), 829.
- Osteoporosis Canada. Fast facts.
- Park, S. M., Jee, J., Joung, J. Y., Cho, Y. Y., Sohn, S. Y., Jin, S. M., Hur, K. Y., Kim, J. H., Kim, S. W., Chung, J. H., Lee, M. K., & Min, Y. K. (2014). High Dietary Sodium Intake Assessed by 24-hour Urine Specimen Increase Urinary Calcium Excretion and Bone Resorption Marker. Journal of bone metabolism, 21(3), 189–194.
- Pelayo, I, Haya, J, De la Cruz, JJ, et al. (2008) Raloxifene plus ossein-hydroxyapatite compound versus raloxifene plus calcium carbonate to control bone loss in postmenopausal women: a randomized trial. Menopause 15, 1132–1138.
- Rüegsegger, P., Keller, A., & Dambacher, M. A. (1995). Comparison of the treatment effects of ossein-hydroxyapatite compound and calcium carbonate in osteoporotic females. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 5(1), 30–34.
- Schmidt, K. H., Wörner, U. M., & Buck, H. J. (1988). Examination of new bone growth on aluminium oxide implant contact surfaces after oral administration of ossein-hydroxyapatite compound to rats. Current medical research and opinion, 11(2), 107–115.
- Stepan, J. J., Mohan, S., Jennings, J. C., Wergedal, J. E., Taylor, A. K., & Baylink, D. J. (1991). Quantitation of growth factors in ossein-mineral-compound. Life sciences, 49(13), PL79–PL84.
- Straub D. A. (2007). Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition, 22(3), 286–296.
- Sunyecz J. A. (2008). The use of calcium and vitamin D in the management of osteoporosis. Therapeutics and clinical risk management, 4(4), 827–836.
- Szterk, A., Zmysłowski, A., & Bus, K. (2018). Identification of cis/trans isomers of menaquinone-7 in food as exemplified by dietary supplements. Food chemistry, 243, 403–409.
- Theuwissen, E., Teunissen, K. J., Spronk, H. M., Hamulyák, K., Ten Cate, H., Shearer, M. J., Vermeer, C., & Schurgers, L. J. (2013). Effect of low-dose supplements of menaquinone-7 (vitamin K2 ) on the stability of oral anticoagulant treatment: dose-response relationship in healthy volunteers. Journal of thrombosis and haemostasis : JTH, 11(6), 1085–1092.
- Unnanuntana, A., Gladnick, B. P., Donnelly, E., & Lane, J. M. (2010). The assessment of fracture risk. The Journal of bone and joint surgery. American volume, 92(3), 743–753.
- Vatanparast, H., Baxter-Jones, A., Faulkner, R. A., Bailey, D. A., & Whiting, S. J. (2005). Positive effects of vegetable and fruit consumption and calcium intake on bone mineral accrual in boys during growth from childhood to adolescence: the University of Saskatchewan Pediatric Bone Mineral Accrual Study. The American journal of clinical nutrition, 82(3), 700–706.
- Wang, H., Bua, P., & Capodice, J. (2014). A comparative study of calcium absorption following a single serving administration of calcium carbonate powder versus calcium citrate tablets in healthy premenopausal women. Food & nutrition research, 58, 10.3402/fnr.v58.23229.
- West, S. L., Swan, V. J., & Jamal, S. A. (2010). Effects of calcium on cardiovascular events in patients with kidney disease and in a healthy population. Clinical journal of the American Society of Nephrology : CJASN, 5 Suppl 1, S41–S47.
- Zhang, Y., Liu, Z., Duan, L., Ji, Y., Yang, S., Zhang, Y., Li, H., Wang, Y., Wang, P., Chen, J., & Li, Y. (2020). Effect of Low-Dose Vitamin K2 Supplementation on Bone Mineral Density in Middle-Aged and Elderly Chinese: A Randomized Controlled Study. Calcified tissue international, 106(5), 476–485